Abstract
Background: Many patients with myelodysplastic syndromes (MDS) have anemia at diagnosis. Erythropoiesis-stimulating agents (ESAs), such as recombinant erythropoietin (EPO), remain the first-choice treatment for anemia in most International Prognostic Scoring System (IPSS) lower-risk MDS patients without del(5q). EPO (~40,000-60,000 U/week) yields ∼60% erythroid responses, as defined by the International Working Group (IWG) 2006 response criteria (Cheson et al. Blood 2006), when baseline EPO is low (<500 U/L) and transfusion requirement is absent/limited. Responses to ESAs occur mostly within 8-12 weeks of treatment. Addition of the oral iron chelator deferasirox (DFX) at low doses could lead to synergism, with elimination of free iron and reduction of toxic reactive oxygen species, improving the bone marrow environment, and enhancing hematopoietic function and response to EPO. Here we present results of a randomized, open-label, Phase II pilot study that evaluated low-dose DFX+EPO compared to EPO alone on erythroid response.
Methods: Patients with IPSS Low- or Int-1-risk MDS (not secondary to treatment with radiotherapy, chemotherapy, and/or immunotherapy for malignant or autoimmune diseases; duration ≥3 months to <3 years), hemoglobin (Hb) ≥8-<10 g/dL, history of <10 RBC transfusions, serum ferritin >300-<1500 ng/mL, and endogenous EPO <500 U/L were enrolled. Patients were randomized 1:1 to DFX (dispersible tablet [DT; 10 mg/kg/day] or, once available, film-coated tablets [FCT; 7 mg/kg/day]) in combination with subcutaneous EPO (40,000 U/week, escalated to 60,000 U/week after 4 weeks if inadequate erythroid improvement) or EPO for 24 weeks (non-responders to EPO after 12 weeks could switch to DFX+EPO for a further 12 weeks). The primary endpoint was the difference in the proportion of patients achieving an erythroid response according to IWG 2006 response criteria (increase from baseline in Hb ≥1.5 g/dL) within 12 weeks. Secondary endpoints included evaluation of hematological responses, tolerability, and safety over 24 weeks.
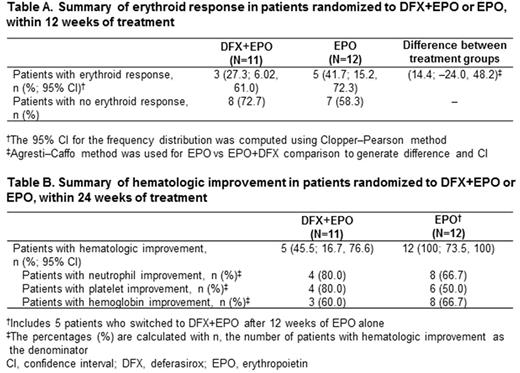
Results: Enrollment was stopped early because of recruitment issues. 23 MDS patients (mean age 72.9 [range 57.7-82.7] years; 43.5% male) were randomized to DFX+EPO (n=11) or EPO (n=12). Eight (34.8%) patients received prior transfusions (n=2 and n=6 in the DFX+EPO and EPO arms, respectively). Baseline serum ferritin was >500 ng/mL in 7 patients in each arm (n=14, 60.9%). After 12 weeks, 5 patients receiving EPO switched to DFX+EPO. Overall, 5 and 4 patients in the DFX+EPO and EPO arms, respectively discontinued; most commonly (n≥1) due to adverse events (AEs; n=4 and n=2). Median EPO exposure was 91 days in patients receiving EPO only (n=7; median dose 270,400 units/week); 77 days in patients receiving DFX DT+EPO (n=10; 295,227 units/week); 100 days in patients receiving DFX FCT+EPO (n=1; 338,800 units/week); and 161 days in patients who switched to DFX+EPO (n=5; 365,833 units/week). Median DFX exposure was 81 days in patients receiving DFX DT+EPO (median dose 10.3 mg/kg/day); 161 days in patients receiving DFX FCT+EPO (3.4 mg/kg/day); and 77 days in patients who switched to DFX+EPO (9.6 mg/kg/day). After 12 weeks, there were 3 (27.3%) erythroid responders in the DFX+EPO arm and 5 (41.7%) in the EPO arm (Table A). Hematologic improvement within 24 weeks was observed in 5 (45.5%) and 12 (100%) patients randomized to DFX+EPO and EPO treatment, respectively (Table B).
The most common AEs (n≥3) over 24 weeks were: gastrointestinal disorders (n=5), and infections/infestations (n=3) in the DFX+EPO arm; musculoskeletal and connective tissue disorders (n=3) in the EPO alone arm; infections/infestations (n=4), general disorders, and administration-site conditions (n=3), and musculoskeletal and connective tissue disorders (n=3) in patients who switched to DFX+EPO. No deaths were reported.
Conclusions: In this small Phase II, pilot study combining low-dose DFX (10 mg/kg/day) with EPO for the treatment of anemia in Low and Int-1 risk MDS patients, there was no beneficial effect on erythroid response. The combination was well tolerated, with no new or unexpected AEs. As reported by Meunier et al. (ASH 2016), higher erythroid proliferation rates were observed in vitro with lower (3 µM; ~5 mg/kg/day) vs higher (>5 µM) DFX concentrations. It remains of interest to investigate outcomes with lower DFX doses in combination with EPO, as well as the use of DFX after EPO failure.
Gattermann: Takeda Pharmaceutical International Co.: Research Funding; Novartis: Honoraria, Other: Travel support; Celgene: Honoraria. DeBonnett: Novartis: Employment. Azmon: Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal